Best Wishes for 2025 and Year in Review!
Best Wishes for 2025 and Year in Review!

🌟 A Year Full of Success and Innovations!
As 2025 begins, we want to share with you the highlights of 2024 that have shaped our journey. Thank you for being part of this incredible story. 🙏
2024 was a year rich in new collaborations and recognition of our expertise...
Renewal of our CIR and CII Certifications
Official recognition of our scientific expertise and a fiscal advantage for our clients for expenses incurred with us in their research and innovation projects.
European Project TETRIS
We are delighted to be the industrial partner of an ambitious project aimed at developing new predictive models for breast cancer care. More details coming soon.
ISO 27001 Certification
A major step forward, confirming our commitment to secure the management of sensitive data and attesting to our expertise in serving our clients.
The year was marked by significant successes and achievements across all activities.
Renewed Trust and New Installations
Thank you to our clients for their continued trust and for adopting our solutions.
In 2024, five new sites were installed. With over 200 installations in France, we strengthen our position as a leader in internal quality control software for medical devices.
Significant Growth in Our Platforms and Solutio
15 new studies launched in 2024, consolidating our presence in oncology clinical trials. Our platform Onco Place experienced remarkable growth, with over 1,000 new users and 300 new investigator centers in 2024 alone. .
A big thank you to the sponsors who trust us in managing their studies.
A Promising New Platform!
Three projects were signed right after its launch, opening exciting opportunities to incorporate patient feedback into studies.
HypoRT Project: Selected as part of the AO UNIRIV program, this innovative project is a turning point in assessing prostate cancer treatments by collecting and analyzing real-life data from over 3,000 patients in France.
Our products and services are now referenced on major national platforms: UNICANCER, RESAH, and UGAP.
Redoubling Our Efforts to Innovate
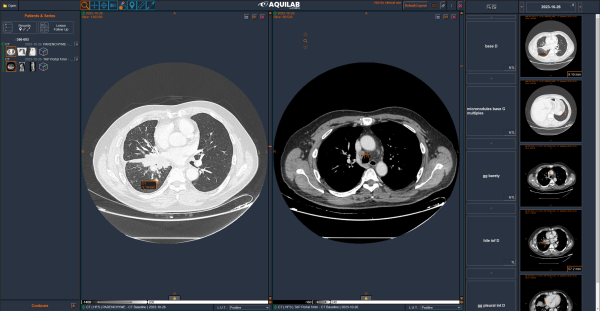
We launched our new online imaging review platform, a modern and intuitive solution to optimize patient monitoring in clinical trials.
ARTISCAN User Forum: An initiative to strengthen exchanges with our clients, improve our solutions, and address user needs.
If you’re not yet a member, feel free to contact our support team.
2024 also saw the publication of numerous studies where we were partners, including the results of the RTEP7 study, recently published in Lancet Oncology.
To prepare for the future, we also launched two new collaborative research theses:
- With GORTEC: To develop the automation of quality control (QC) in radiotherapy clinical trials.
- With the Oscar Lambret Center: To develop new methodologies to enhance reliability in the use of quantitative MRI.
An Ambitious Strategy for 2025
2025 promises to be an exceptional year:
- International Development is at the heart of our strategy, promoting our QA and clinical trial activities in new markets.
- ARTISCAN will undergo numerous upgrades to support new equipment and test objects.
- In clinical trials, we will launch our Centralized Imaging Review offering, an optimized solution developed in partnership with our radiologist partners.
The entire AQUILAB team wishes you a successful, healthy, and innovative 2025.
Together, let’s continue transforming cancer care through science and innovation.